If during perimenopause and into menopause you feel more ”reactive”, more inflammed, and more emotionally volatile than ever, you need to consider your CELLULAR support.
A lesser-known but critically important structure inside the cells is the Endoplasmic Reticulum (ER).
The ER is a major player in how your body handles:
- Stress
- Inflammation
- Hormone processing
- Metabolic signals
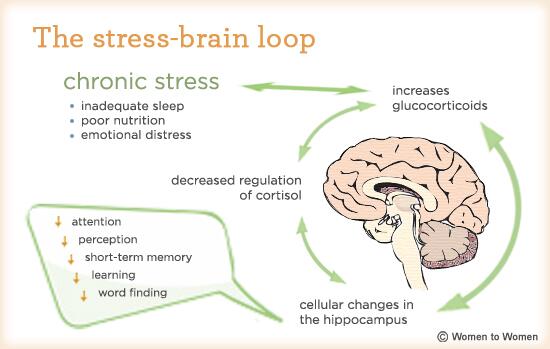
When oestrogen declines during menopause, the ER become less efficient — and this dysfunction can ripple out across your entire body.
What Is the Endoplasmic Reticulum? Why Does It Matter in Menopause?
The Endoplasmic Reticulum (ER) is a network of folded membranes found inside your cells. There are two types:
- Rough ER is studded with ribosomes and helps fold and transport proteins — including enzymes and hormones.
- Smooth ER handles lipid metabolism, detoxification, calcium storage, and steroid hormone processing.
In short? The ER is your “cellular stress manager”. It ensures that every protein your body makes is folded correctly, packaged properly, and sent to the right place.
But when the ER is overwhelmed, by inflammation, toxins, or declining oestrogen, it starts to malfunction. Proteins misfold. Stress signals escalate. The body shifts into a low-grade alarm state that disrupts everything from sleep and metabolism to mood and energy.
And during menopause, this cellular stress response can become supercharged.
How Menopause Disrupts ER Function
During your reproductive years, oestrogen plays a protective role throughout your body, including at the cellular level. It helps regulate inflammation, supports mitochondrial function, helps your ER stay resilient under stress.
But as oestrogen levels decline in the menopausal transition, your ER loses one of its key allies. And the consequences ripple out in powerful ways.
Increased ER Stress
Without enough oestrogen, the ER becomes more vulnerable to:
- Oxidative stress
- Environmental toxins
- Blood sugar fluctuations
- Chronic inflammation
This creates a buildup of misfolded or “unfinished” proteins, which triggers something called the Unfolded Protein Response (UPR). While this response is designed to restore balance, when overactivated, it can lead to cellular dysfunction, inflammation, and even cell death.
For a research paper on the topic – enter this link into your browser:
https://www.mdpi.com/1422-0067/26/18/8814